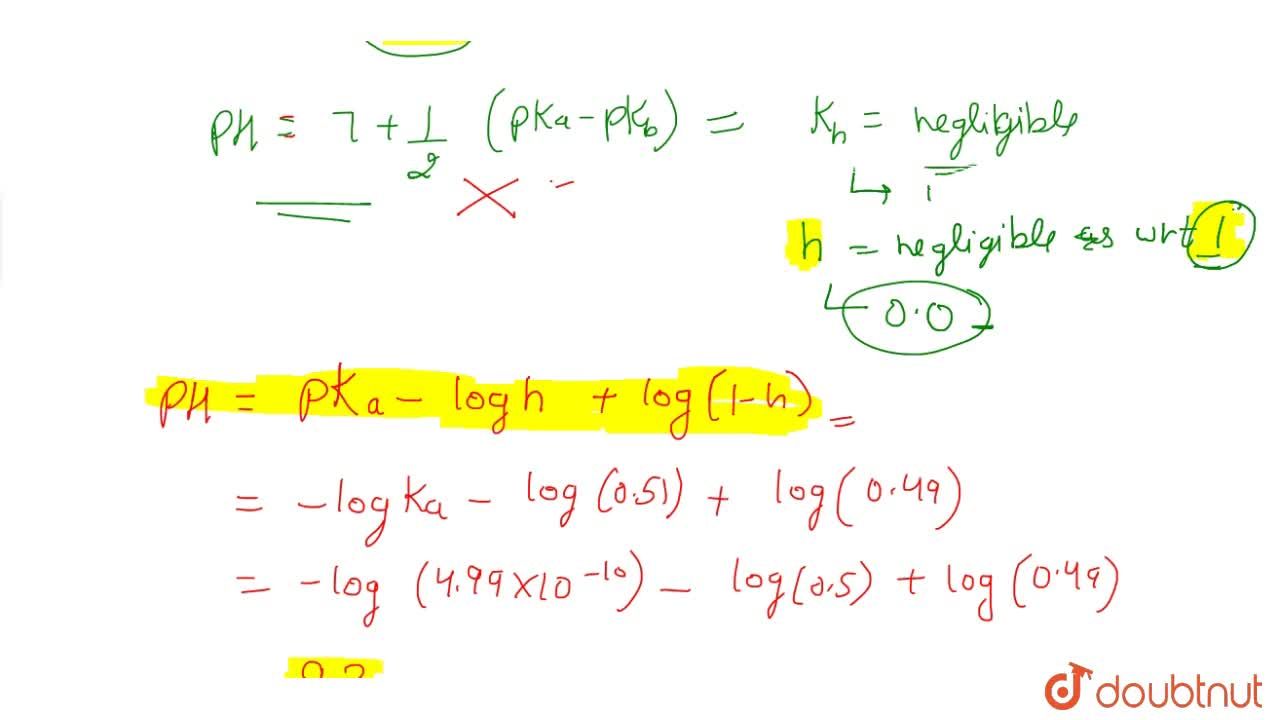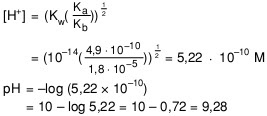
SOLVED:Calculate the pH of a solution prepared from 0.200 mol of NH4CN and enough water to make 1.00 L of solution.

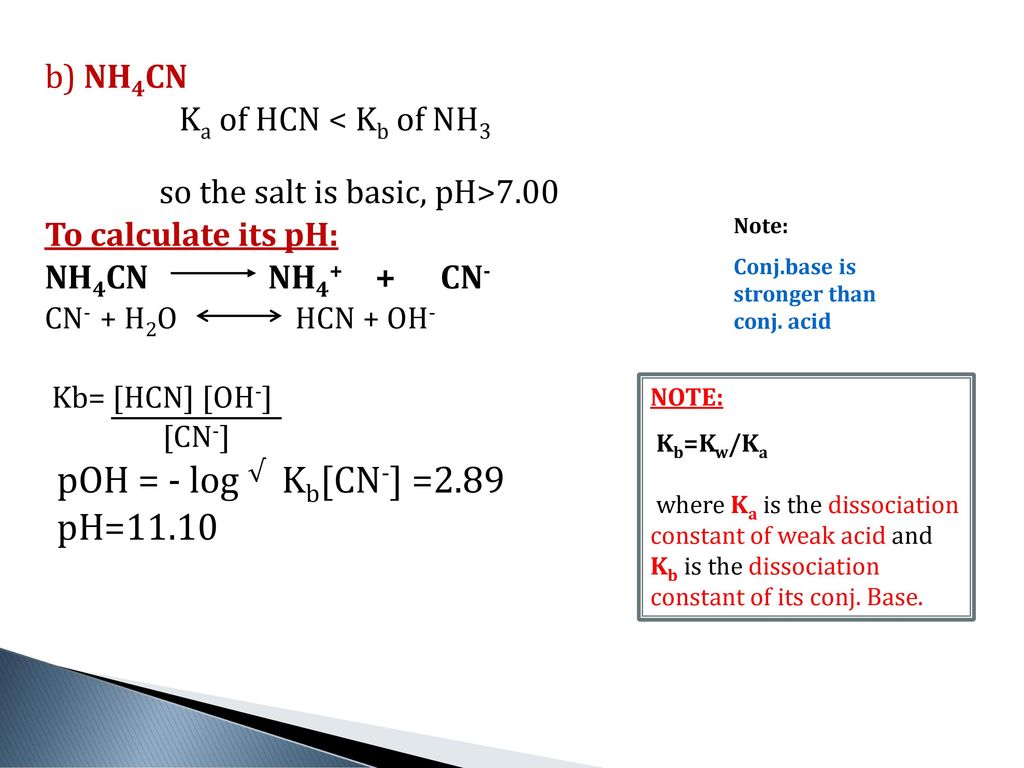
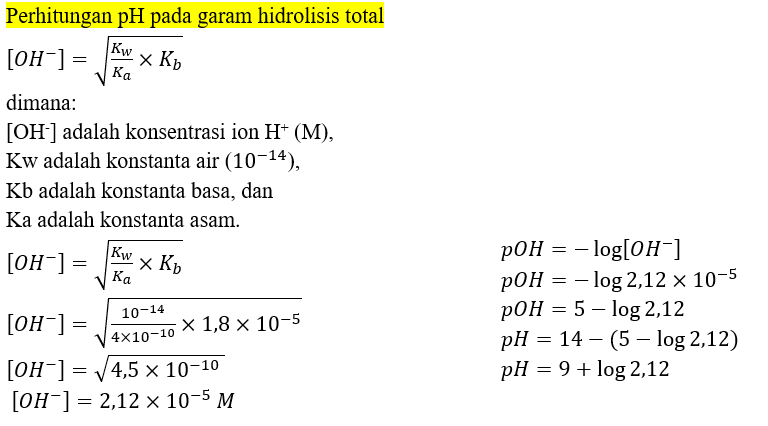
Calculate the pH of 0.01 M solution of NH4CN . The dissociation constants Ka for HCN = 6.2 × 10^-10 and Kb for NH3 = 1.6 × 10^-5 .

Calculate the pH of 0.01 M solution of NH4CN . The dissociation constants Ka for HCN = 6.2 × 10^-10 and Kb for NH3 = 1.6 × 10^-5 .
pH of 0.1 M solution of NH_4CN solution is x. If its concentration is increased to 0.2 M then pH of the solution will be (1) 2x (2) x/2 (3) x (4) x/4

Calculate the pH of 0.01 M solution of NH4CN. The dissociation constants ka for HCN=6.2×10^-10 and kb - Brainly.in

Polymers | Free Full-Text | Tuning the Morphology in the Nanoscale of NH4CN Polymers Synthesized by Microwave Radiation: A Comparative Study

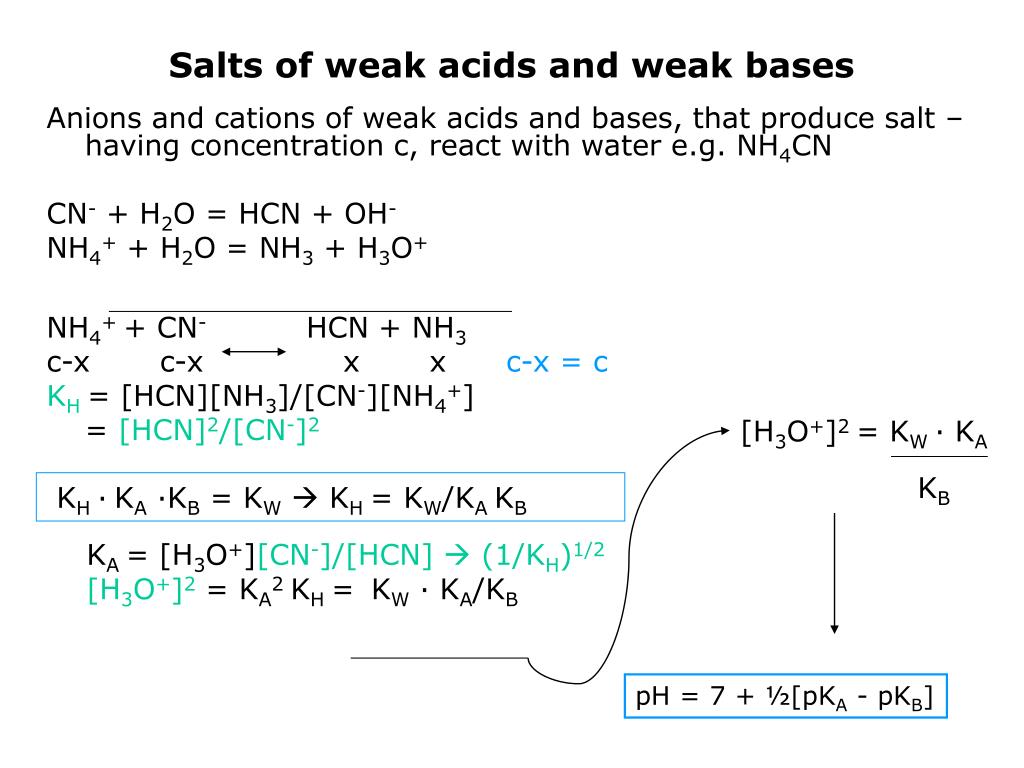
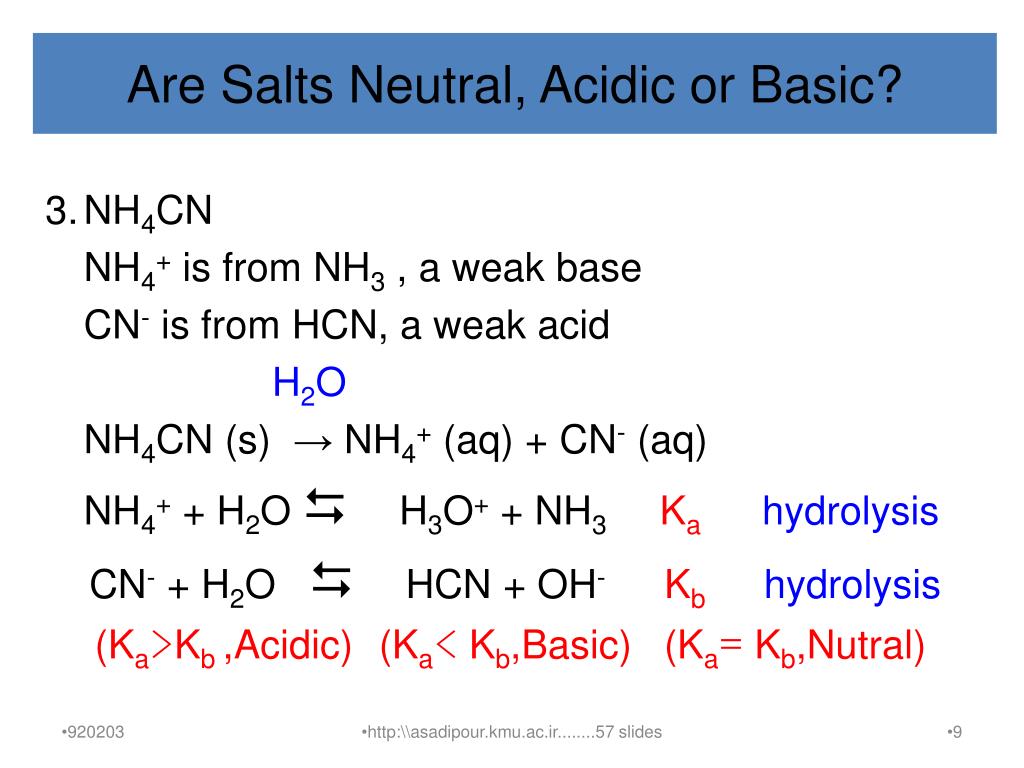
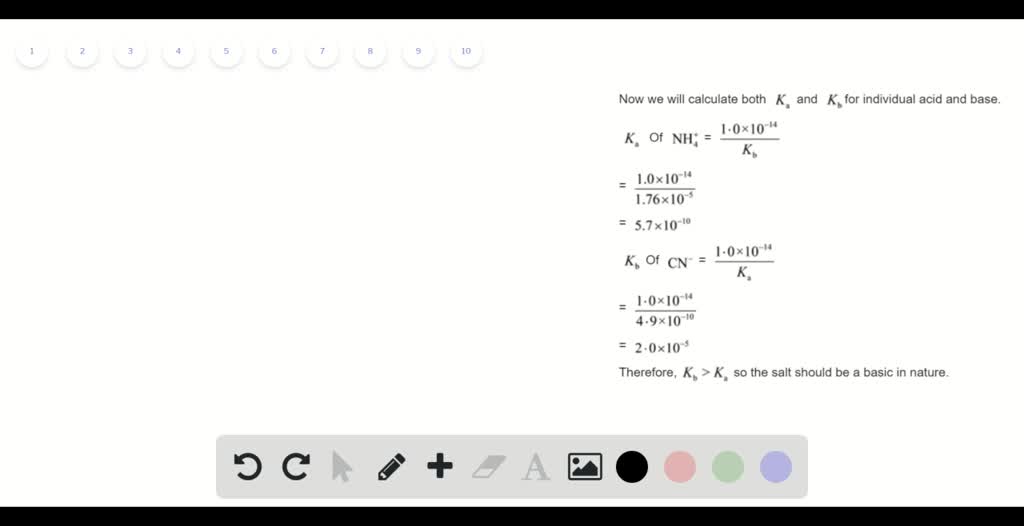
aqueous solution - Accurate method to calculate the pH of a salt from a weak acid and weak base - Chemistry Stack Exchange