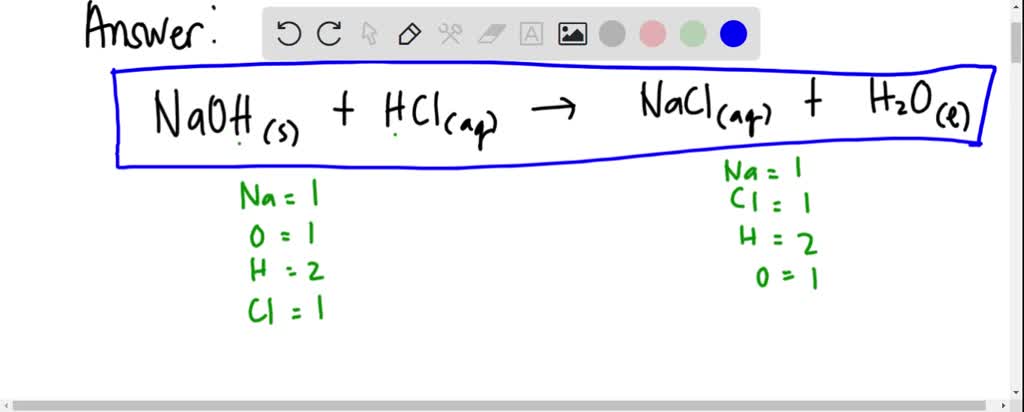
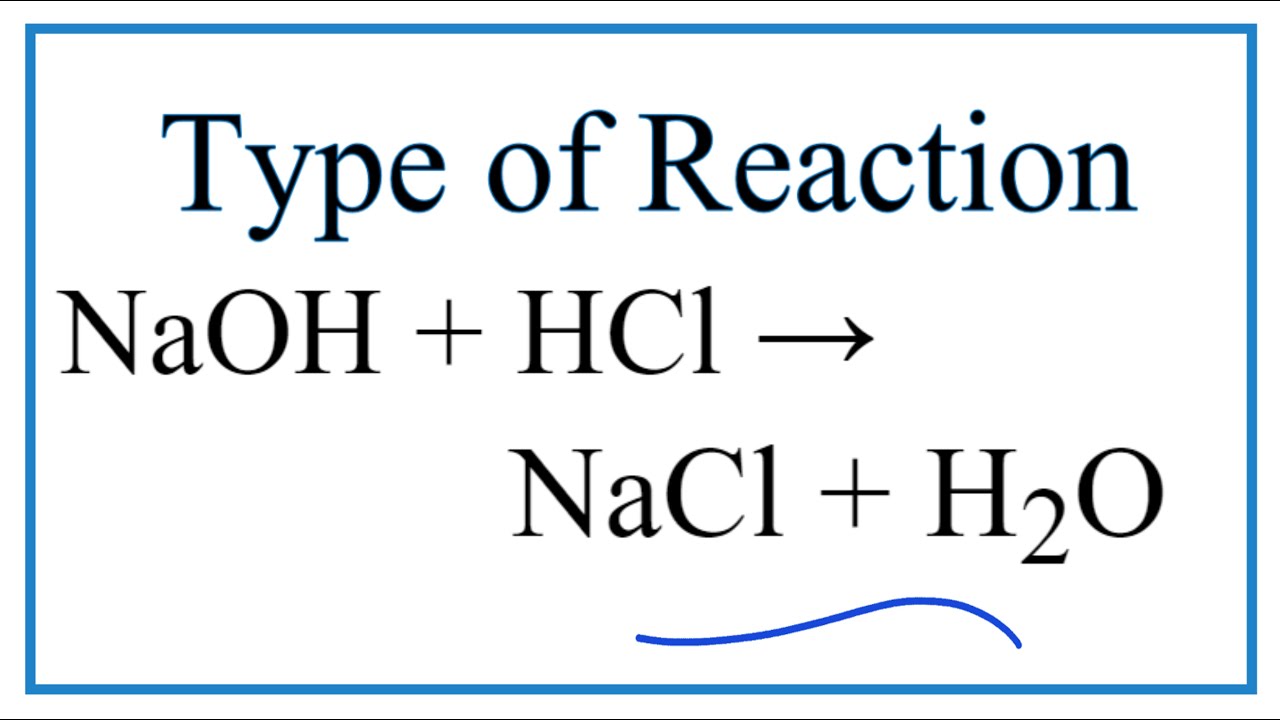
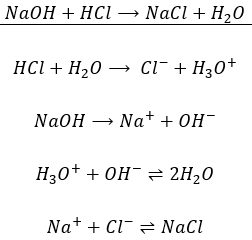Write the neutralization reaction between Hydrochloric acid HCI and sodium hydroxide NaOH, and write the equation for this process.
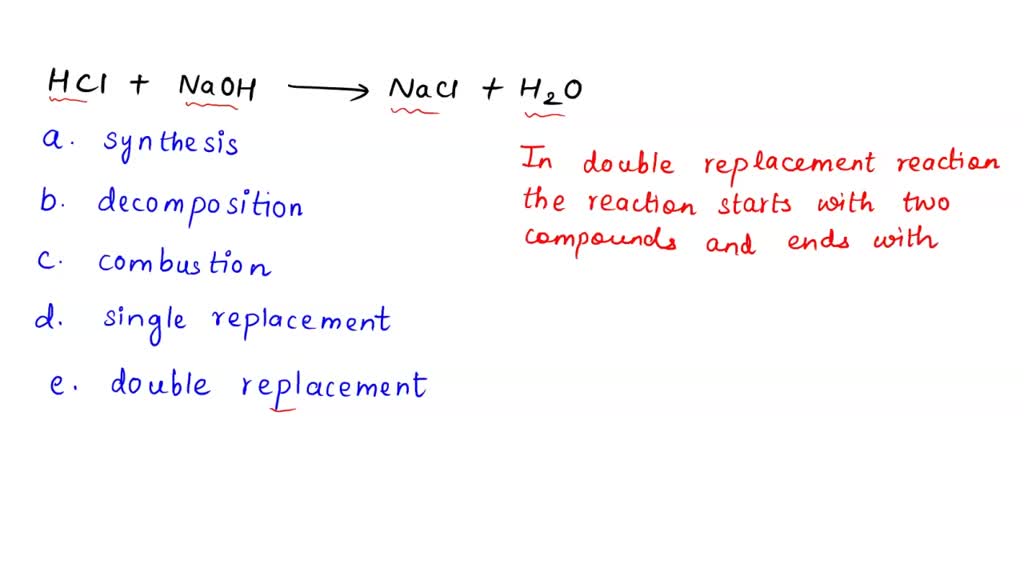
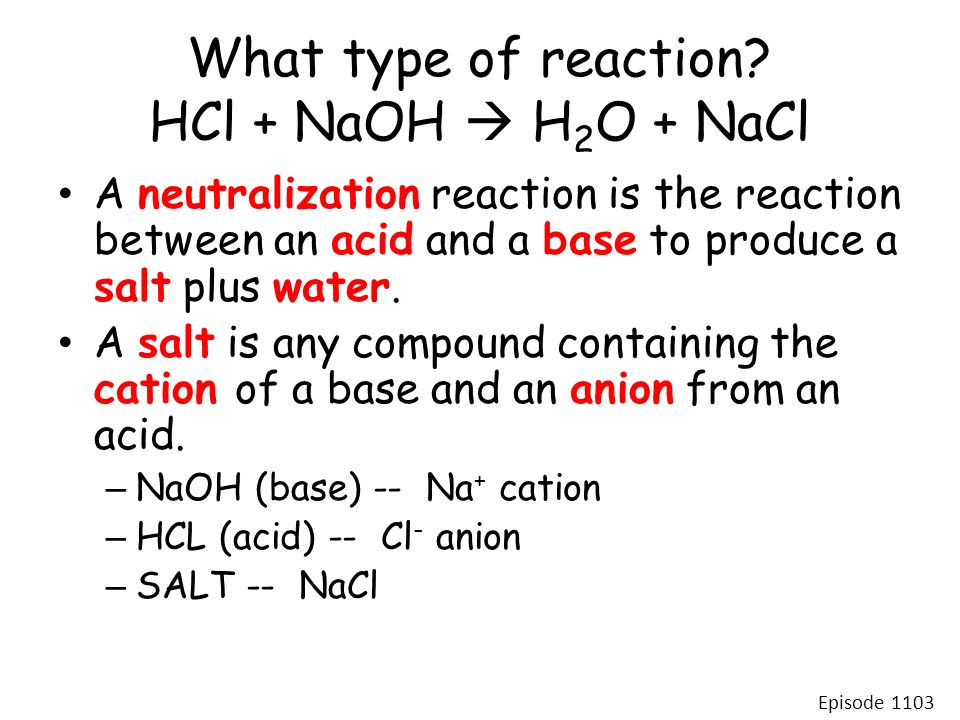
SOLVED: HCl + NaOH —> NaCl + H2O What is the main classification of this reaction? a.) synthesis b.) decomposition c.) combustion d.) single replacement e.) double replacement

Set Of Three Chemical Containers With Acid Base And Salt With Different Ph Hcl Hydrochloric Acid Naoh Sodium Hydroxide And Nacl Sodium Chloride Stok Vektör Sanatı & Kimya'nin Daha Fazla Görseli -