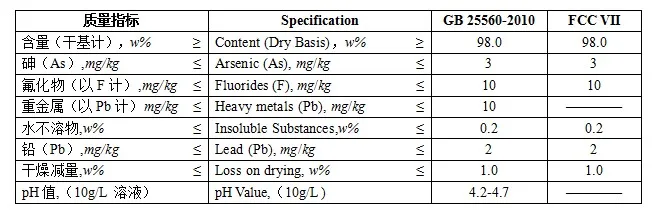
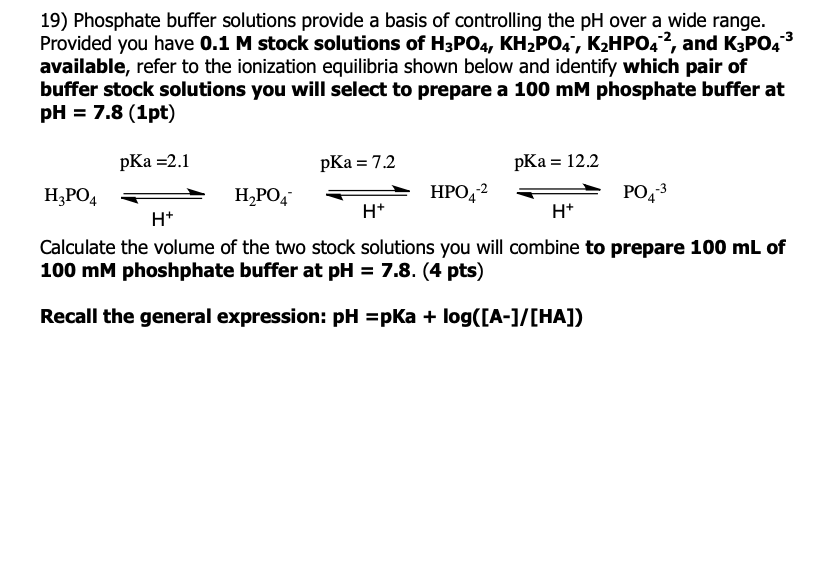
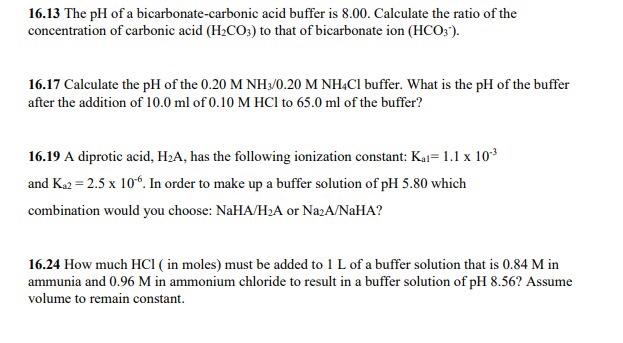
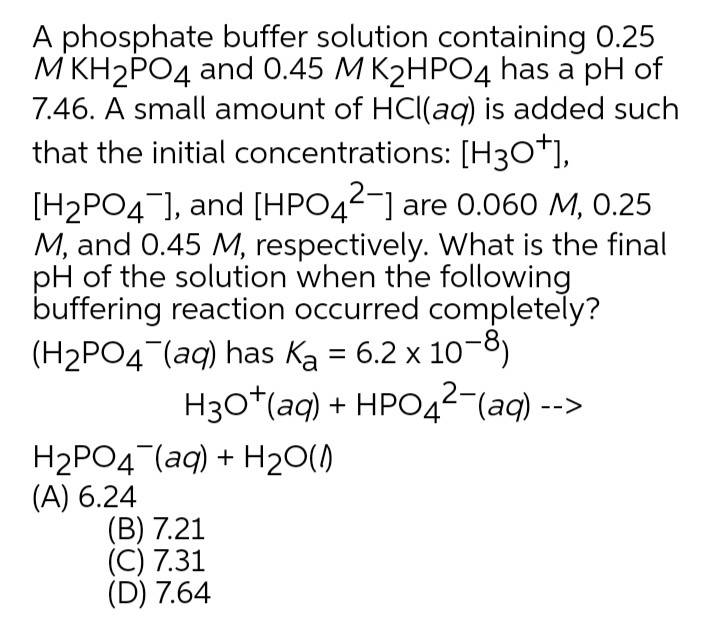
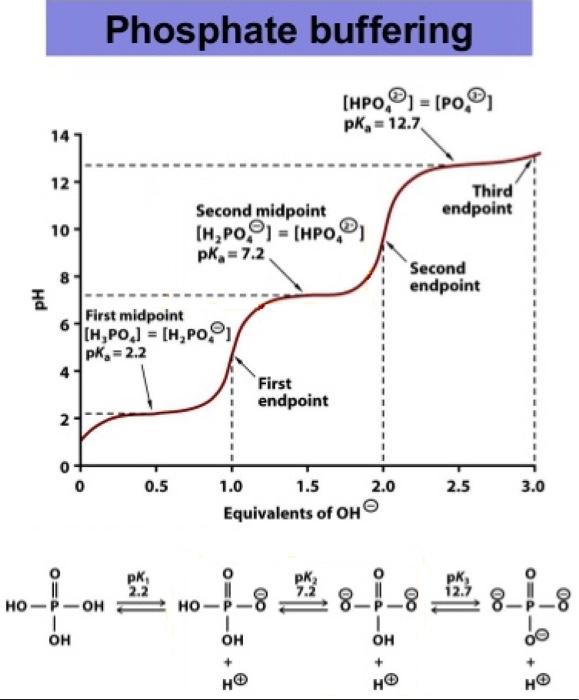
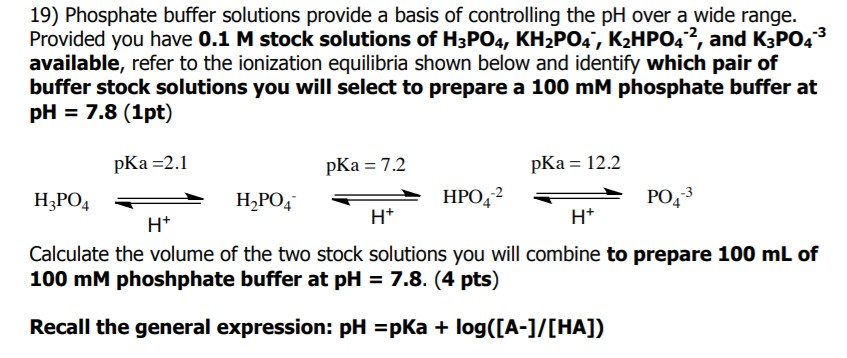
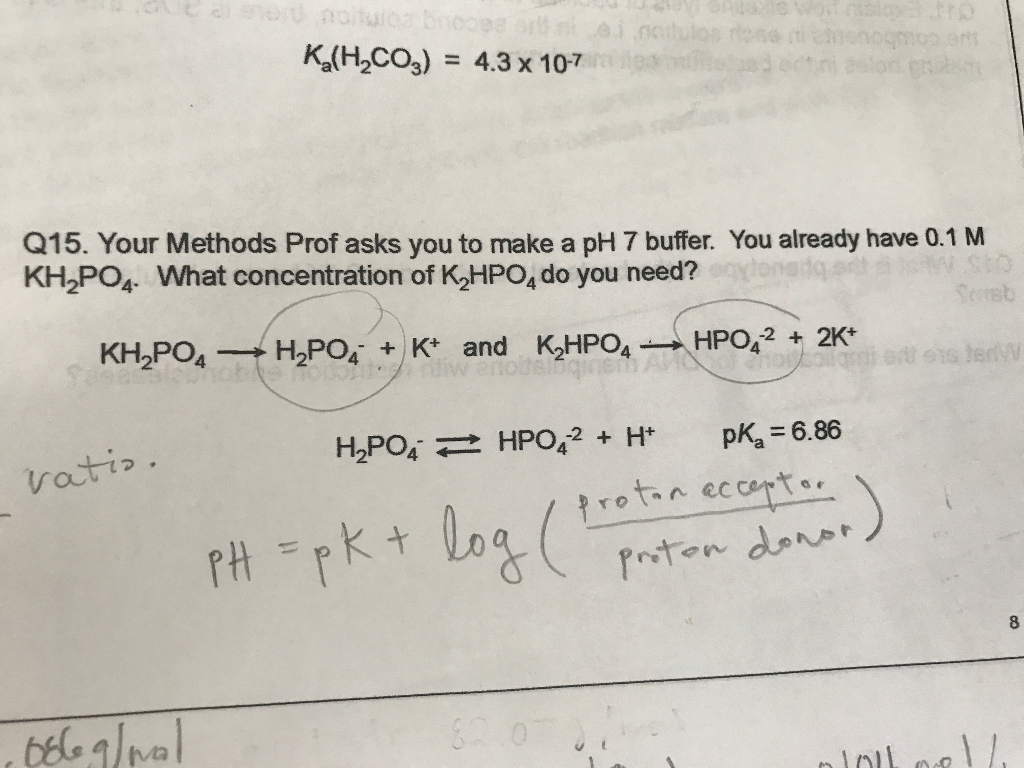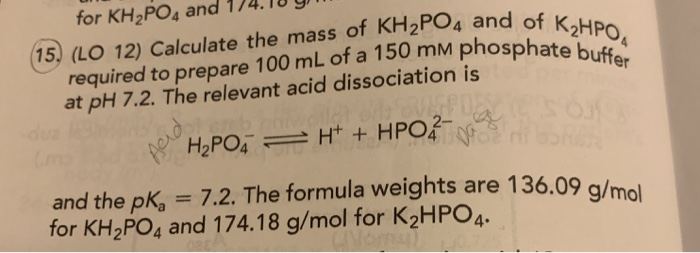Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com

CD spectra at 20 °C in potassium phosphate buffer (10 mM KH2PO4/K2HPO4,... | Download Scientific Diagram

SOLVED: In order to create a buffer zone at physiological condition (neutral pH) the potassium phosphate buffer is to be made of: K3PO4 and KzHPO4 KzHPO4 and KH2PO4 KHzPO4 and HzPO4 KzHPO4

Nitrogen loss reduction by adding KH2PO4-K2HPO4 buffer solution during composting of sewage sludge - ScienceDirect
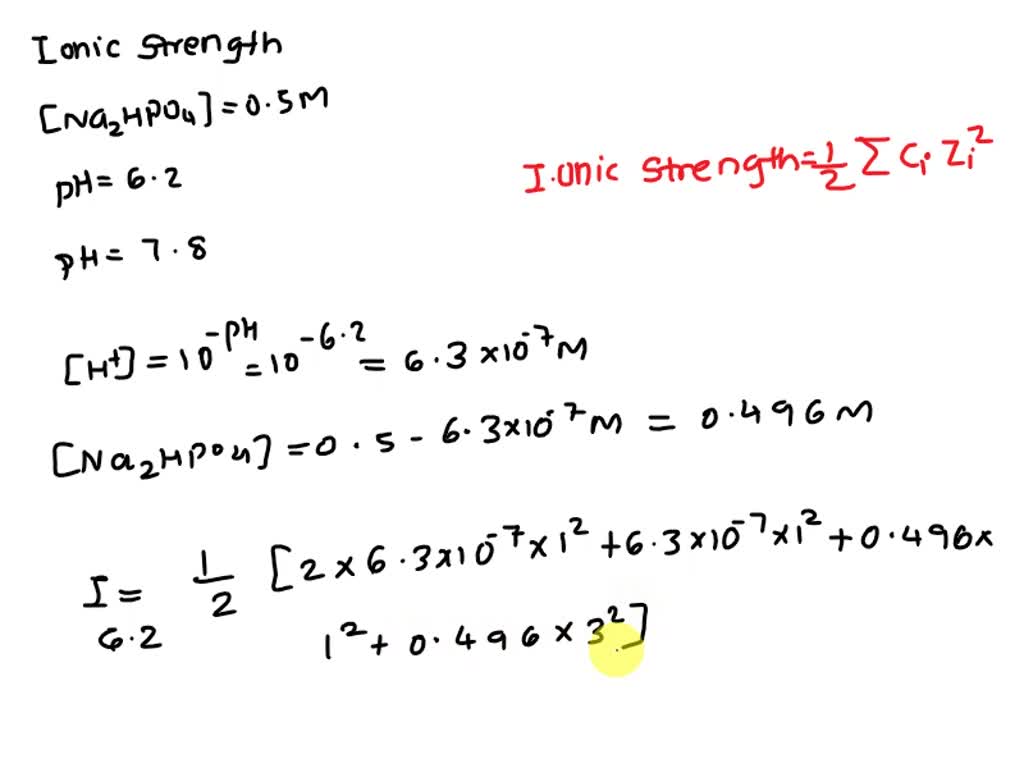
SOLVED: A buffer solution was prepared by mixing 0.1M K2HPO2 and 0.1M KH2PO4 (pH 6.64) - calculate the ionic strength (I) of the standard phosphate buffer solution
![SOLVED: What is the pH of the buffer system made up of 0.10 M Na2HPO4/0.15 M KH2PO4? [ H2PO4- dissociation constant Ka = 6.2x10-8] 8.6 1.5 10.0 None of these SOLVED: What is the pH of the buffer system made up of 0.10 M Na2HPO4/0.15 M KH2PO4? [ H2PO4- dissociation constant Ka = 6.2x10-8] 8.6 1.5 10.0 None of these](https://cdn.numerade.com/ask_previews/a5a00885-2780-4697-a3a6-6966ddf39b81_large.jpg)
SOLVED: What is the pH of the buffer system made up of 0.10 M Na2HPO4/0.15 M KH2PO4? [ H2PO4- dissociation constant Ka = 6.2x10-8] 8.6 1.5 10.0 None of these
Effect of concentration (a) Tris-HCl buffer pH 6.5 at dilution of urea... | Download Scientific Diagram

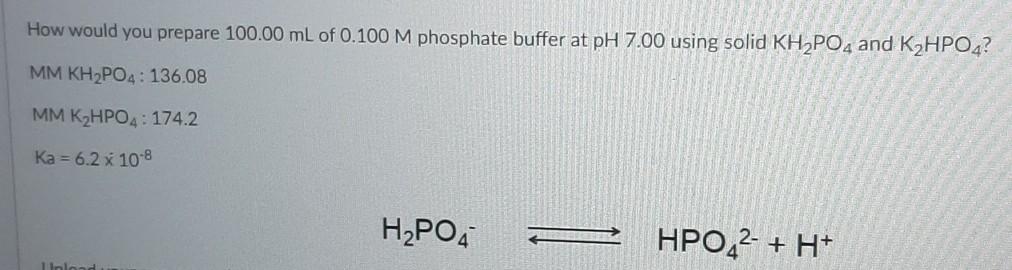
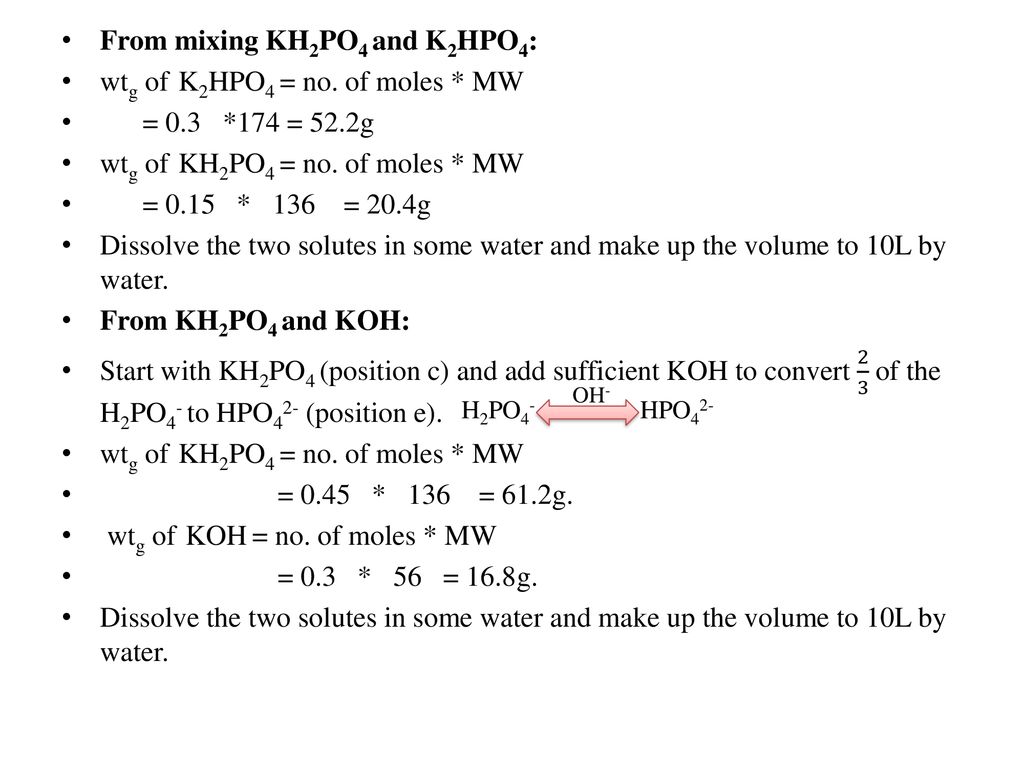
SOLVED: Using the Henderson-Hasselbalch equation and a pKa of 7.21, determine the mass (grams) of KH2PO4 and K2HPO4 that you will require to prepare 500 mL of the phosphate buffer solution to

The effect of feeding buffer solutions (3.0 mol m−3 K2HPO4/KH2PO4) of... | Download Scientific Diagram

OneClass: A buffer solution at pH = 7.00 prepared with KH2PO4 (pKa=7.21) and Na2HPO4 has a total phos...