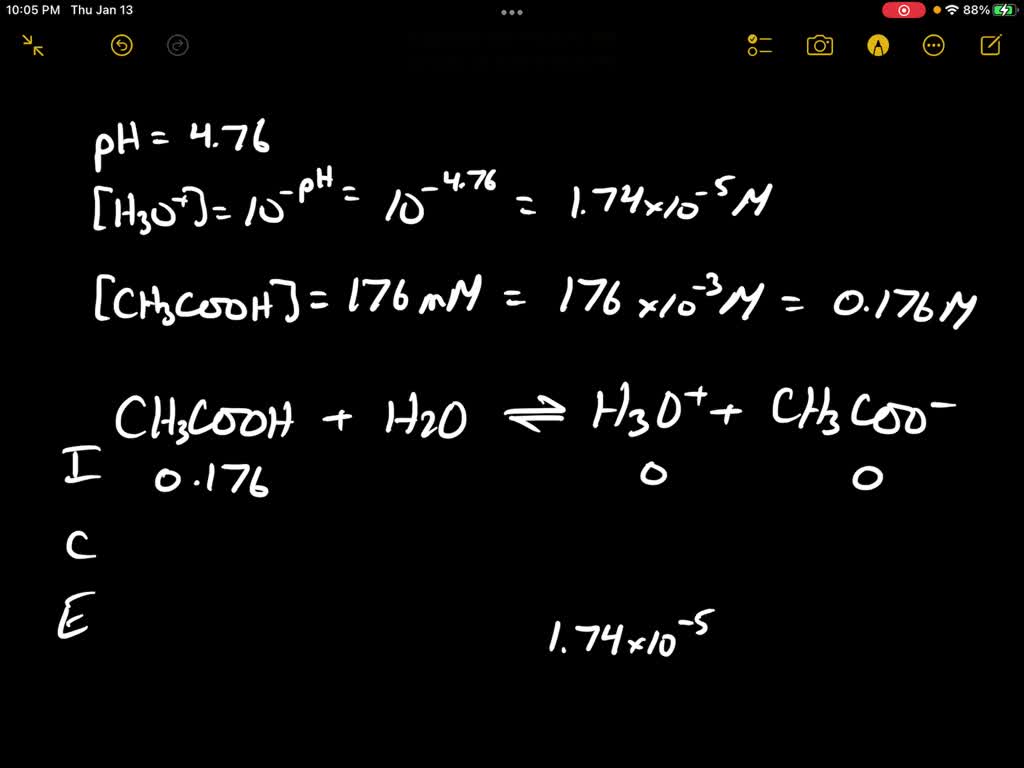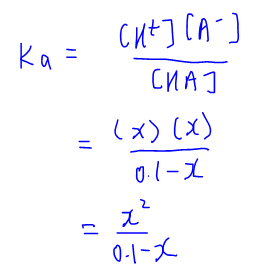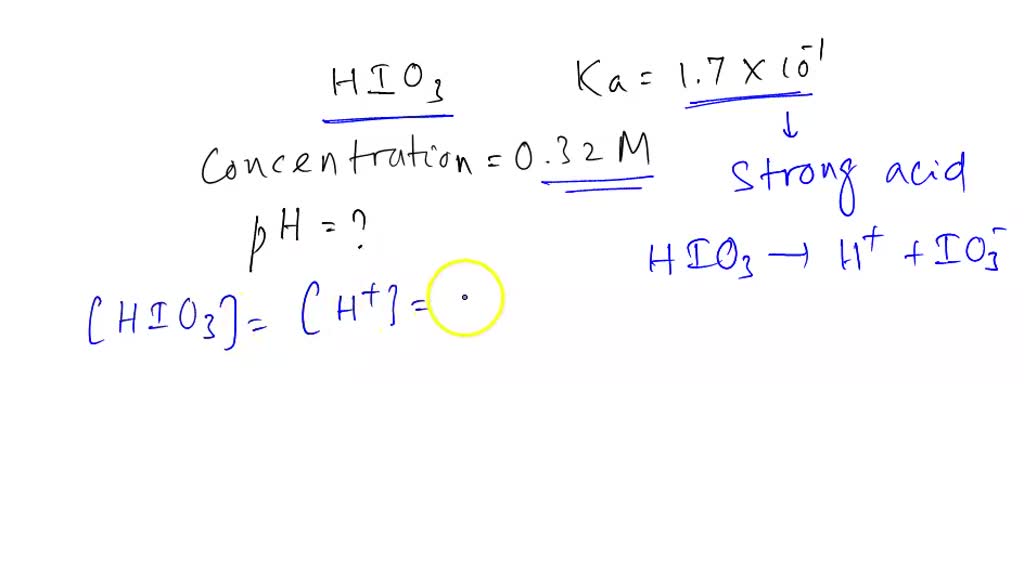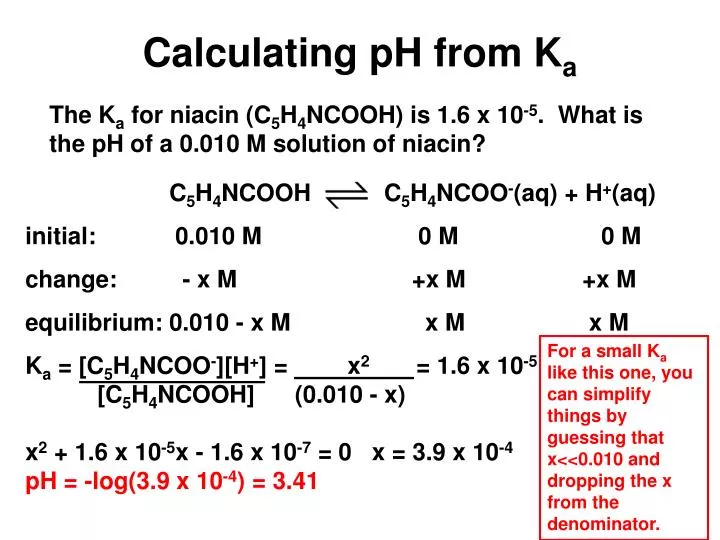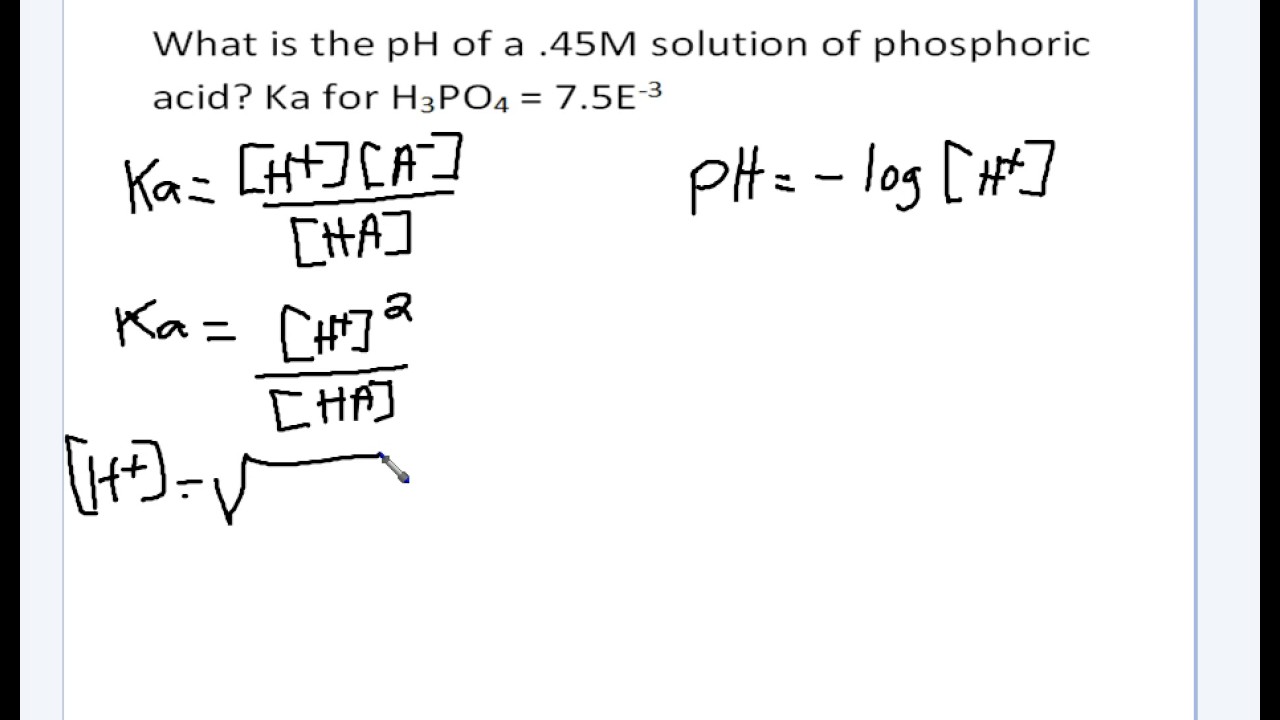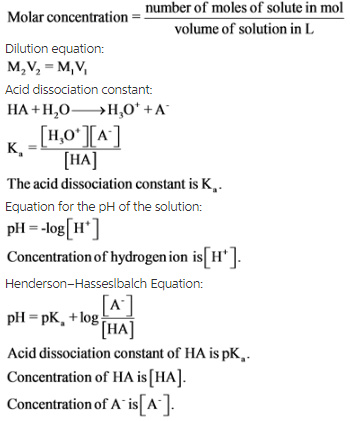
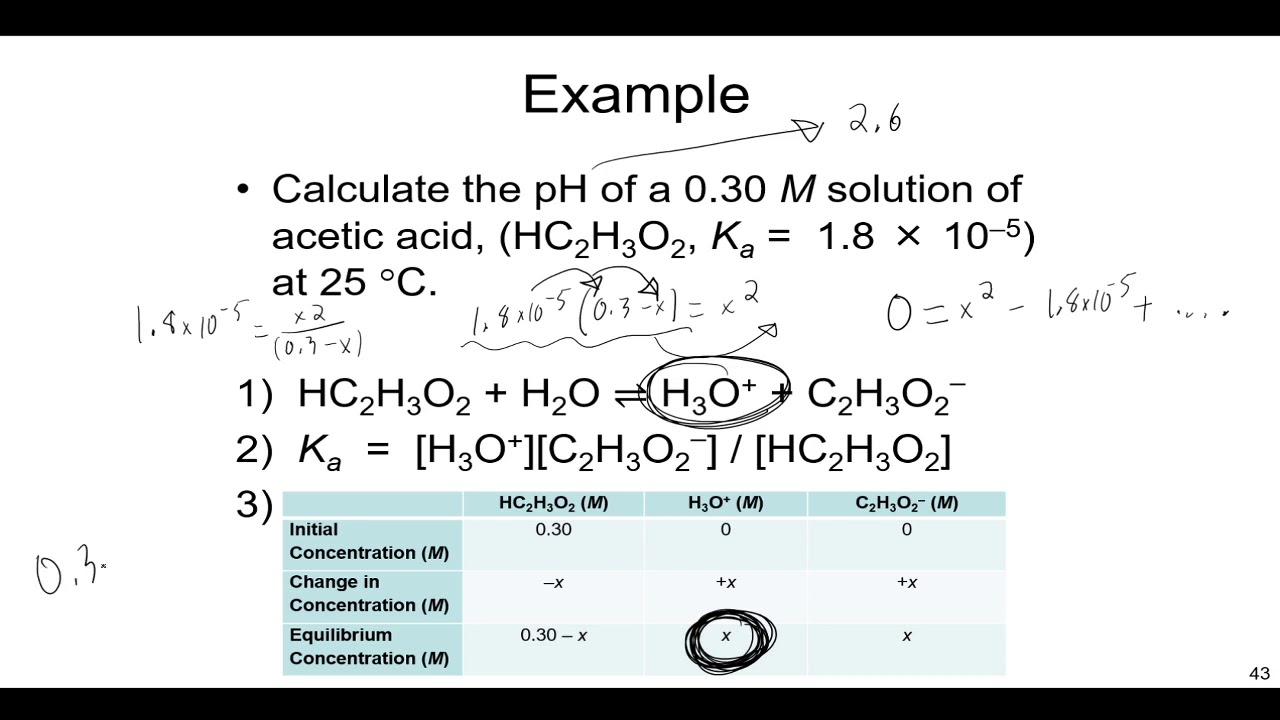
The Ka value for acetic acid, CH3COOH(aq), is 1.8x10^-5. Calculate the ph of a 2.80 M acetic acid solution - Home Work Help - Learn CBSE Forum

The Ka for lactic acid, HC3H5O3 is 8.3 x 10^{-4}. What is the pH of a 0.40 M solution of the acid? What is the % ionization of the acid at this

Calculate the pH of the following mixture given Ka = 1.8 × 10^-5 and Kb = 1.8 × 10^-5 ( pKa = pKa = 4.7447 ) 50mL 0.05M NaOH + 50mL of 0.1M CH3COOH

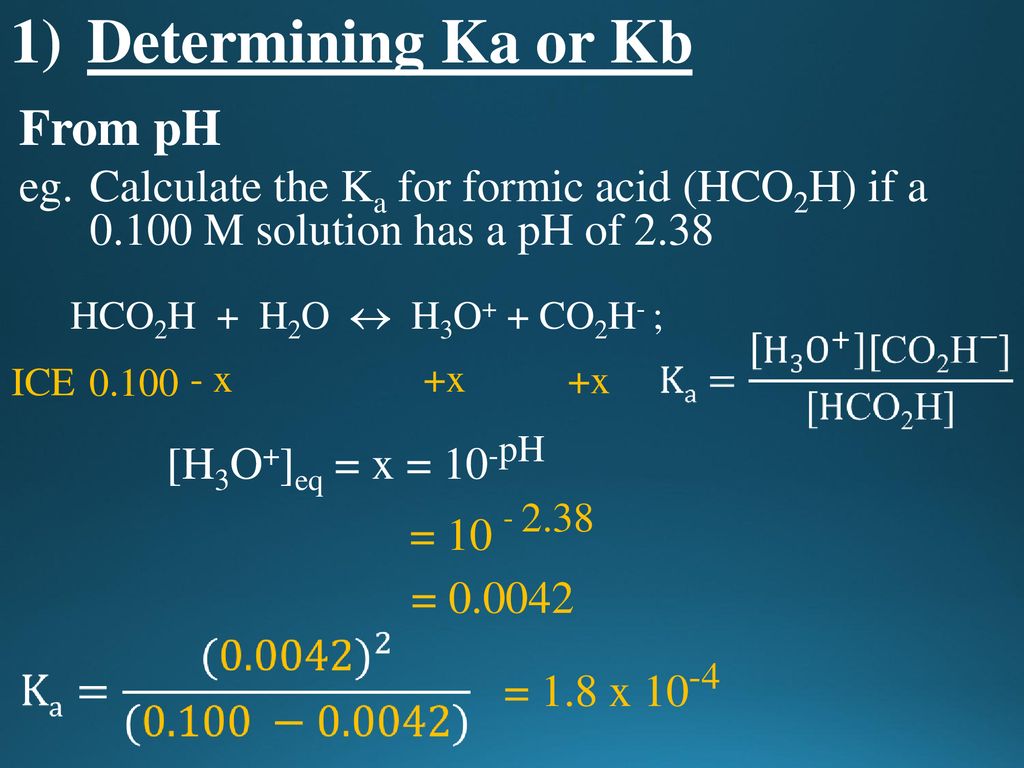
![pH, [H+], Ka and pKa calculations | Teaching Resources pH, [H+], Ka and pKa calculations | Teaching Resources](https://dryuc24b85zbr.cloudfront.net/tes/resources/6298743/image?width=500&height=500&version=1386765493000)

![Solved 3. Calculate the [H3O+], [OH-], pH and pOH of a 0.120 | Chegg.com Solved 3. Calculate the [H3O+], [OH-], pH and pOH of a 0.120 | Chegg.com](https://media.cheggcdn.com/study/f5c/f5c5a593-319b-4d2b-9ea3-2e5d84e0d782/image)


![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)