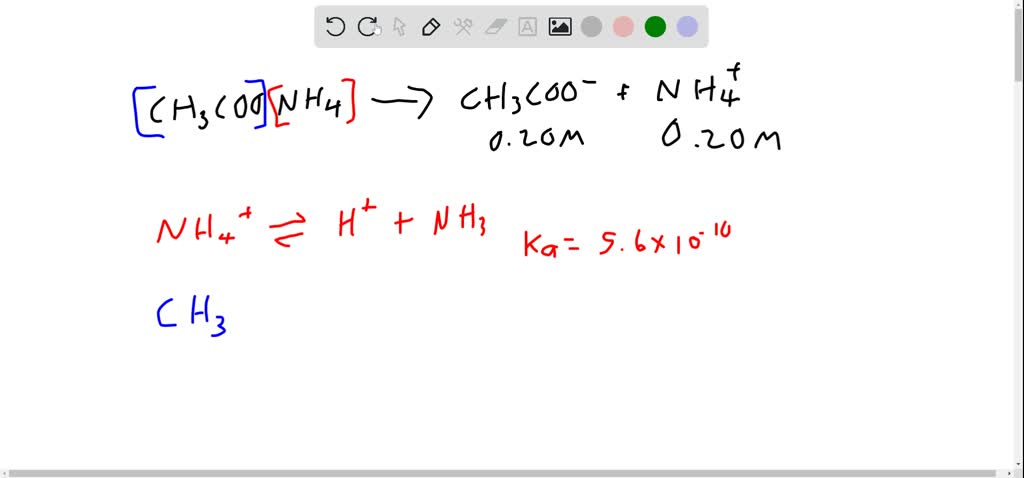
Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

Ammonium acetate which is 0.01 M, is hydrolysed to 0.001 M concentration. Calculate the change in pH - Brainly.in

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.
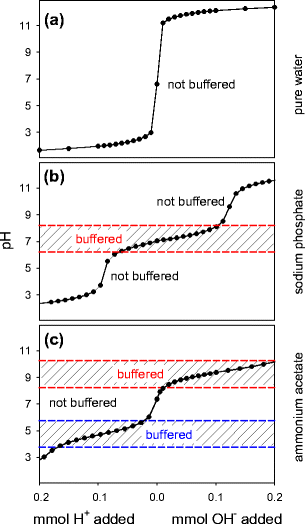
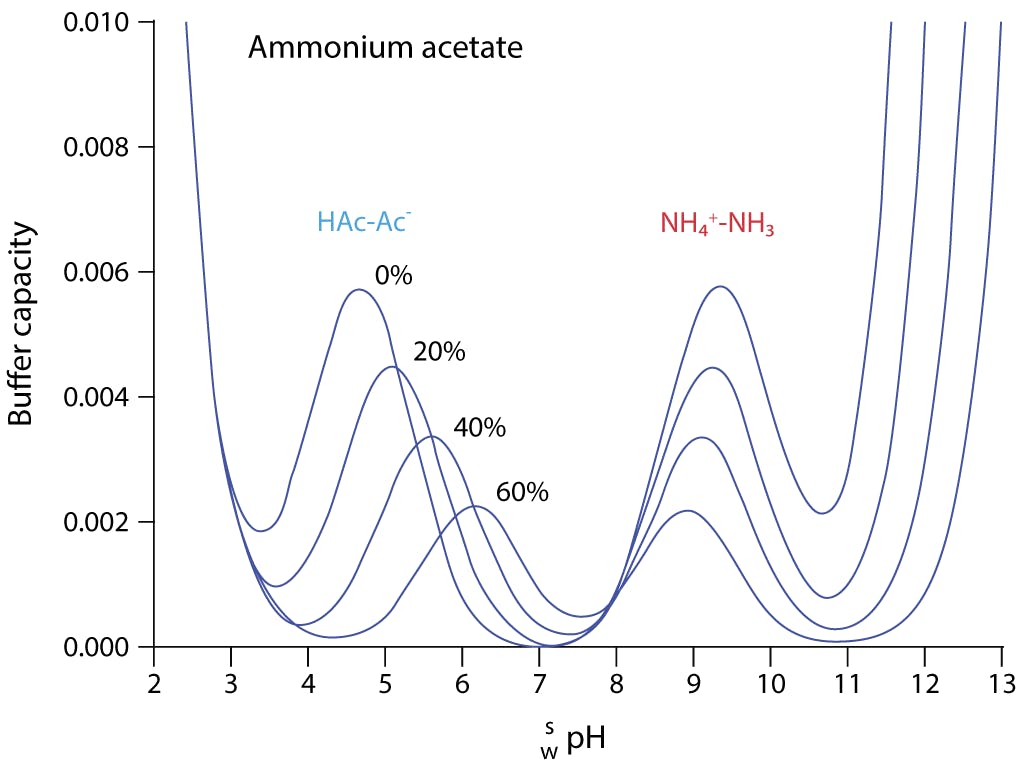
Addressing a Common Misconception: Ammonium Acetate as Neutral pH “Buffer” for Native Electrospray Mass Spectrometry | SpringerLink

Addressing a Common Misconception: Ammonium Acetate as Neutral pH “Buffer” for Native Electrospray Mass Spectrometry | Journal of the American Society for Mass Spectrometry
Identity correct statement (a)degree of hydrolysis decrease on doubling the concentration of aqueous solution of CH3COONH4 (b)for 1M CH3COOH pH=pKa/2 (c)salt hydrolysis depends on size of atom (d)all

SOLVED: Find the pH of 0.20 M ammonium acetate solution given the values below (Kc for NH; 18*10 K; of HCH;CO,H 8K1O ) pkh (C4,co2)t-w) 04 (c4sCo ) 17 (CIlg Cot A)aA)

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.
![Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ] Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ]](https://d1hj4to4g9ba46.cloudfront.net/questions/1200266_999624_ans_e02120991ce8408c94c67fa9a8ab69a7.jpg)

![pH of a solution of 0.1 M [CH3COONH4(aq)] is [given: Ka(CH3COOH) = Kb(NH4OH) = 1.8 x 10^-5)] pH of a solution of 0.1 M [CH3COONH4(aq)] is [given: Ka(CH3COOH) = Kb(NH4OH) = 1.8 x 10^-5)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/644382825_web.png)