a pH versus blank-corrected titration volume (0.1 M HCl) for PAAconf.b... | Download Scientific Diagram
100 mL of 0.1 M HCl is taken in a beaker and to it 100 mL of 0.1 M NaOH is added in steps of 2 mL and the pH is continuously
![50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ] 50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/OHZYS2ZrRHZKams=/sd/)
50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ]
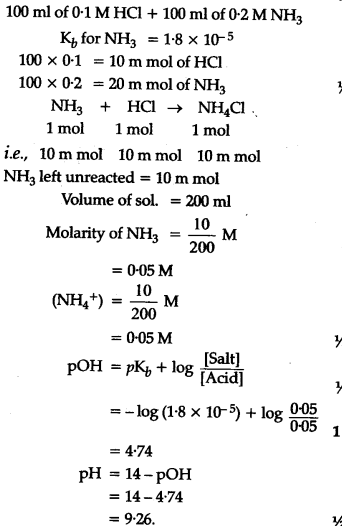
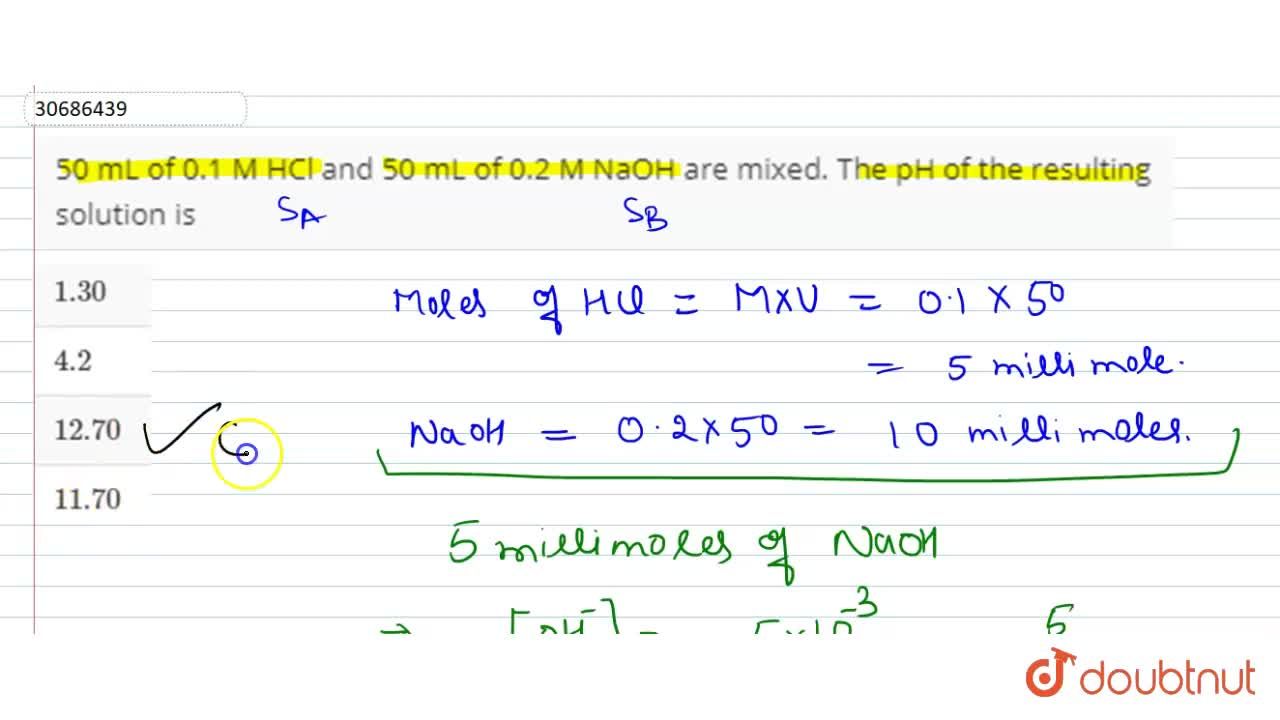
Calculate the pH of a solution obtained by mixing of 100 ml of 0.1 M HCl and 100 ml of 0.2 M - CBSE Class 11 Chemistry - Learn CBSE Forum

if equal volumes of 0.1 M H2SO4 and 0.1 M HCl are mixed then the pH of resulting solution will be (log 15 = 1.176)

SOLVED: 1. Calculate the pH if you added 3 mL of 0.1 M HCL to a) 97 mL of pure water at pH 7, and b) 100 mL of phosphate buffer (0.063
What is the pH of the resulting solution when the equal volume of 0.1M Noah and 0.01M HCL are mixed? - Quora
Calculate pH of [HS^–], [S^2–], [Cl^–] in a solution which is 0.1 M HCl & 0.1 M H2S given that Ka1 (H2S) = 10^–7, Ka2 (H2S) = 10^–14 - Sarthaks eConnect | Largest Online Education Community

A solution contains 0.09 M HCI , 0.09 M CHCl2COOH , and 0 .1 M CH3COOH . The pH of this solution is 1 . K for CHCl2COOH is 1.25 × 10^-x . The value of x is :[Given : Ka for CH3COOH = 10^-5 )

What is the pH of a solution containing 50ml of 1M HCl and 100ml of 0 25M HCl Answer - Chemistry - Equilibrium - 13180741 | Meritnation.com